

Althéra® HMO
Whey based extensively hydrolyzed, nutritionally complete hypoallergenic speciality formula with 2 HMO (2’-FL and LNnT) and purified lactose for effective first-line symptom relief in cow’s milk
protein allergy (CMPA).
A sole source of nutrition from birth to 12 months of age. Supplement in combination with age-appropriate complementary foods from around 6 months of age.
Advancing the
Management of
Cow’s Milk
Protein Allergy
Preparing Althéra® HMO is very similar to how you prepare standard infant formula. The step-by-step instructions on how to prepare Althéra® HMO are:

STEP 1 Wash hands thoroughly before preparation.
STEP 2 Wash bottle, teat and cap.

STEP 3 Boil bottle for for 5 min. Leave covered until use.

STEP 4 Boil drinking water for 5 min, allow to cool.

STEP 5 Pour exact amount of lukewarm water into bottle as per feeding table on tin.

STEP 6 Add exact number of level scoops for age of babyas per feeding table on tin.

STEP 7 Shake bottle until powder fully dissolved. Use immediately and do not keep unfinished bottle, discard contents.

STEP 8 Close tin tightly after each use and store in a cool, dry place. Must be used within 3 weeks of opening.
WARNING “Unboiled water, unsterilsed bottles or incorrect dilution can make your baby ill. Incorrect storage, handling, preparation and feeding can also lead to adverse effects. Follow instructions carefully.”
| AGE | QTY PER MEAL (ML) | QTY PER MEAL (SCOOPS) | MEALS PER DAY |
|---|---|---|---|
|
1-2 weeks
|
90
|
3
|
6
|
|
3-4 weeks
|
120
|
4
|
7
|
|
2nd month
|
150
|
5
|
5
|
|
3-4 months
|
180
|
6
|
5
|
|
5-6 months
|
210
|
7
|
5
|
|
From 5th (7th) month**
|
210
|
7
|
3-4
|
lactose, maltodextrin, vegetable oils (suflower, rapeseed, coconut),extensively hydrolized whey protein (milk), minerals (calcium glycerophosphate, potassium phosphate, magnesium chloride, calcium chloride, sodium chloride, manganese sulfate, ferrous sulphate, potassium iodide, sodium phosphate, zinc sulphate, potassium citrate, potassium chloride, sodium selenate, copper sulphate), emulsifier (E472c), fibres (2'-fucosyllactose, lacto-N-neotetraose), mortierella alpina oil (ARA), oil from the micro-algae Schizochytrium sp.(DHA), acidity regulator (E330), choline bitartrate, vitamins (C, E, niacin, pantothenic acid, riboflavin, A, thiamin, B6, folic acid, K, D, biotin, B12), L-arginine, L-histidine, Taurine, Inositol, L-Carnitine.

NE = Niacin Equivalent
DFE = Dietary folate equivalent
DHA = Docosahexaenoic acid
ARA = Arachidonic acid
Osmolarity = 273 mOsm/l
Osmolality = 305 mOsm/kg
- Proven hypoallergenicity with a tolerance of 98.4% in infants with cow’s milk protein allergy (CMPA)1
- Provides immune enhancing properties, with the addition of 2 HMO (2’-FL and LNnT†) and purified lactose to support the growth of beneficial bacteria and the production of important short chain fatty acids2,3
- Reduces the risk of common respiratory-tract infections as well as medication use in infants with CMPA through the addition of 2’-FL and LNnT† to formula2
- Supports growth and development in infants and young children with CMPA2 – commonly at risk of growth failure and nutritional deficiencies4,5
- Inspired by breastmilk’s specific amino acid profile and with a protein quantity of 9 PE%
- Helps compliance with preferred taste over some casein based extensively hydrolyzed formulas6
- Highest degree of hydrolysis amongst extensively hydrolyzed formulas7
†LNnT = Lacto-N-neotetraose; 2’-FL= 2’-Fucosyllactose; HMO = Human milk oligosaccharides.
Immune-Nurturing Benefits
CMPA is an immune-mediated condition. It is associated with gut microbiota dysbiosis, which impacts immune system maturation and leaves infants at an increased risk of infections and future allergies.8 Althéra® HMO contains lactose, as well as 2’-fucosyllactose (2’-FL) and lacto-N-neotetraose (LNnT). 2FL and LNnT are two significant human milk oligosaccharides (HMO) that are structurally identical to those found in breastmilk.9
HMO are bioactive components that support the infants in the following ways.10,11




Reducing infections and medication use

Infants fed formula with 2’-FL and LNnT† had fewer upper and lower respiratory tract infections12

Infants fed formula with 2’-FL and LNnT† had lower antibiotic and antipyretic use13
Patient Case Example For Althéra® HMO
NAME & AGE
Edward, 3 months old
DIAGNOSIS
Cow’s Milk Protein Allergy
DIETARY MANAGEMENT
Althéra® HMO

Case Presentation: Edward
Edward, a 3-month-old boy – was exclusively breastfed for the first 2 months – occasionally topped up with standard infant formula. He experienced frequent episodes of regurgitation/ vomiting, crying (which was very stressful for parents, especially mum) and occasionally loose stools. A family history of atopy (mother) was noted, and he was referred for assessment by a gastroenterologist (GI) by the general practitioner (GP). His CoMiSS score was 16 (crying 6; regurgitation 6; loose stools 4).
Gastro-Esophageal Reflux Disease (GERD) at 4 months of age. Prescribed Proton Pump Inhibitors (PPIs). Suspected cow’s milk protein allergy (CMPA).
Seen by dietician who recommended skin prick tests (SPT) to cow’s milk (CM). PPIs appeared to help reduce the regurgitation/vomiting symptoms but without full resolution. His weight gain was poor (birth weight 3.4kg weight-for-age z score (WAZ) 0 = normal), current weight 3.6kg (WAZ close to -2). He was prescribed Althéra HMO for a trial period of 2-4 weeks. His SPT results were negative. The dietician also advised that he start dairy-free complementary feeding (CF) between 4-6 months of age.
After 1 week mum called to report that Edward was crying less and seemed to be more settled, though still some vomiting and loose stools.
Edward was 5 months of age. His weight had improved – 4.0kg (WAZ again close to 0) - he was regurgitation/vomiting less and his stools were less frequently runny. Mum reported he was much more settled and therefore she was reluctant to challenge him, as she felt her life was getting back to normal, stating ‘’I finally have my baby back’’. They had also started dairy-free CF. The challenge was therefore postponed but agreed with mum to discuss his challenge at 8 months of age. His CoMiSS score was 3-7 (crying 1; regurgitation 2; stools occasionally 4).
On review he was growing well (5.6 kg, WAZ >0 = normal), progressing on CF – and was a ‘’happier baby’’. The GI had discontinued PPIs as he had significantly fewer regurgitation/vomiting episodes. Mum reported that he had had an accidental challenge (cheese) without noticeable symptoms, therefore she was happy to discuss the challenge with the dietician. The reintroduction of dairy/milk using milk ladder at home was advised. His CoMiSS score was now 1 (crying 0; regurgitation 1; loose stools 0). By his 1st birthday he was back on standard formula and taking all dairy.


Introducing Our Plant-Based Packaging Designed to Be Recyclable
Our plant based plastic is made from sugar cane which is a renewable resource unlike fossil based material. Additionally, we have ensured a lighter pigment colour to improve recyclability.
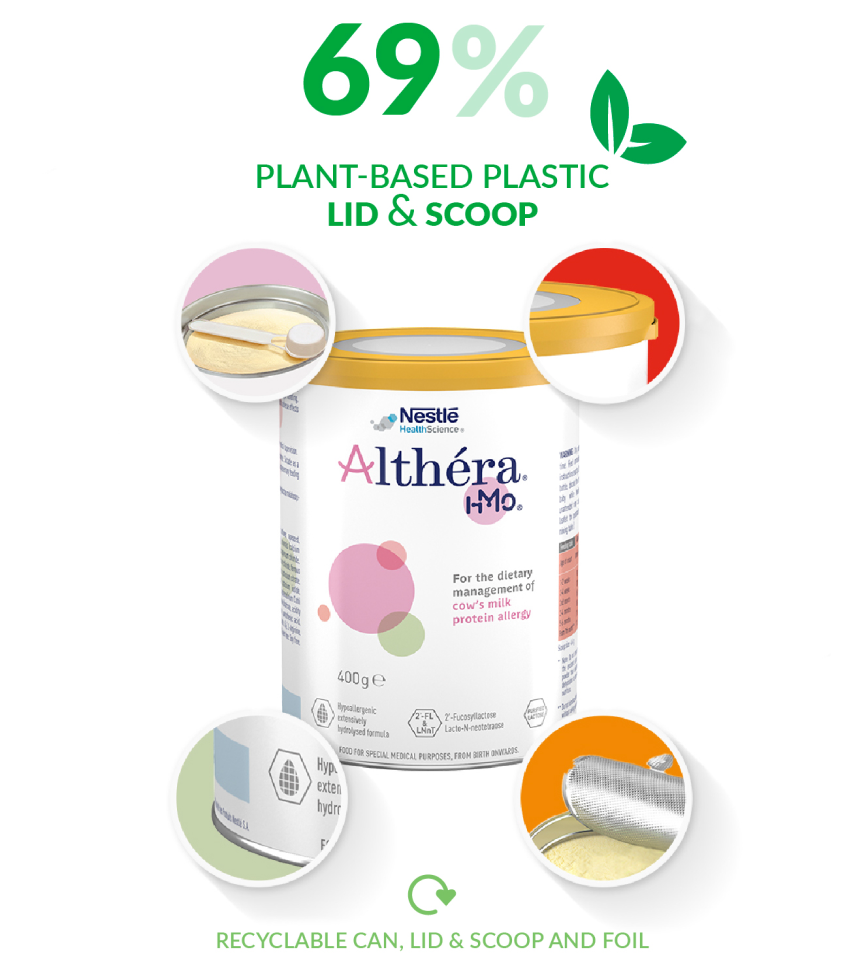
…of the plastic in the Althéra lid and scoop combined are now made with plant-based materials (sugar cane) instead of fossil fuel-based materials.
How We Convert Sugar Cane to Plant Based Plastic
Curious to learn more about how our plant based plastics are typically made?


This is where the resin to make our plant based plastic comes from.

Althéra® HMO FAQs
Answers to common questions
about
Althéra® HMO
.
The tin sized of Althéra HMO is 400g.
It is 4.4g – so 1 scoop of Althéra HMO is equal to 4.4g of powder.
Althéra® HMO is suitable for those following a halal diet (halal certified) but is not kosher certified.1 The protein hydrolyzate used in Althéra® HMO is uniquely produced with non-porcine enzymes.1 These enzymes (proteases) are of plant origin. This formula meets the hypoallergenicity criteria of the American Academy of Pediatrics (AAP) and can be used for the dietary management of infants and young children with CMPA.2
- 1. Data on file (Halal certification).
- 2. Nowak-Wegrzyn A, et al. Allergy 2019;74(8):1582-4.
Yes, Althéra® HMO can be used for infants requiring enteral tube feeding. Instructions need to be followed according to local guidance as well as the patient’s individual nutritional management plan.
Lactose is the largest solid component in breastmilk, representing around 70g/Litre1,2, and breastmilk is the best feed for all infants, including those with CMPA. It has been suggested that lactose provides beneficial effects on gut physiology, including prebiotic effects, as well as stool softening and enhancement of water, sodium and calcium absorption.3 The European Society of Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) position papers (2012 & 2023) considers the complete avoidance of lactose in infants with CMPA to be longer warranted.3,4 Previous concerns that infants with CMPA would react to residual protein traces in lactose have often resulted in complete avoidance of both lactose and cow’s milk protein.4 However, adverse reactions to lactose in infants with CMPA is not supported by literature. ESPGHAN (2012) suggested that formula containing purified lactose have been found to be safe and effective in the treatment of CMPA.3
Francavilla and colleagues (2012) demonstrated that an extensively hydrolyzed formula (eHF) with added lactose confers prebiotic benefits in infants with CMPA.5 They reported that the addition of lactose shaped the composition of the gut microbiota by increasing total fecal counts of Lactobacillus and Bifidobacterium species, while decreasing levels of Bacteroides and Clostridia.5 Furthermore, the addition of lactose has been shown to be associated with better product palatability, which could improve acceptance/compliance.6,7
Althéra® HMO is the only formula in our CMPA (AAA) range which contains lactose. Althéra® HMO has been developed for the first-line management of CMPA and in hypoallergenicity studies was tolerated by 98.4% of infants with CMPA.8-9 Alfaré® HMO, Alfamino® HMO and Alfamino® Junior HMO are lactose-free specialty formulas and are all suitable for secondary (transient) lactose intolerance.
- 1. Zivkovic et al. Proc Natl Acad Sci USA. 2011;108(suppl 1) :4653‐4658.
- 2. Zivkovic et al. Funct Food Rev. 2013;5(1):3-12..
- 3. Koletzko S, et al. J Pediatr Gastroenterol Nutr. 2012;55(2):221-229.
- 4. Vandenplas Y, et al. An ESPGHAN position paper. J Pediatr Gastroenterol Nutr. 2023. https://www.espghan.org/dam/jcr:7100468b-c6df-48bc-a566-6b13c427e756/CMA%20ESPGHAN%202022_V31.pdf;
- 5. Francavilla R, et al. Pediatr Allergy Immunol. 2012;23(5):420-7.
- 6. Maslin K, et al. Pediatr Allergy Immunol.2018;29(8):857-62.
- 7. Miraglia Del Giudice M, et al. Ital J Pediatr. 2015;41:42.
- 8. Nowak-Węgrzyn A, et al. Allergy. 2019;74(8):1582-4.
- 9. Nowak-Wegrzyn A, et al. Nutrients. 2019;11:1447.
HMO structures are quite complex, and the replication of HMO (that are structurally identical, but not sourced from human milk) was not possible until recently.
Advances in biotechnology, after almost 30 years of research and development activities by Nestlé and their partners, enabled the production of HMO, such as 2’fucosyllactose (2’-FL) and lacto-N-neotetraose (LNnT), to be introduced into standard and specialty infant formulas. 2’-FL and LNnT in Nestlé formulas are structurally identical to two of the top 10 HMO in human milk.1
- 1. Bode L & Jantscher-Krenn E. Adv Nutr. 2012;3(3):383S-91S.
More than 200 HMO have been identified in breastmilk,1 with 2’fucosyllactose (2’-FL) and lacto-N-neotetraose (LNnT) being among the 10 most abundant, accounting for more than 30% of all HMO.2 They are well studied and are considered to be safe for use in standard infant formula and in speciality infant formulas by the European Food Safety Authority (EFSA).3
The first clinical studies in healthy infants using standard infant formulas supplemented with 2’-FL and LNnT demonstrated their safety, supporting normal growth, with beneficial effects on immunity, gut microbiota development and the rate of some infections as well as the need for some medications.4-6 Clinical studies in infants with cow’s milk protein allergy (CMPA) showed that our specialty formulas containing 2’-FL and LNnT were hypoallergenic, supported normal growth, effectively alleviated CMPA symptoms, reduced the risk of infections and potentially the need for some medications,7 and positively shaped the gut microbiome.7-10
Infants with CMPA can now benefit from two of the most abundant HMO in human milk, 2’-FL and LNnT, which have been proven to nurture the infants’ immune system, addressing gut microbiota dysbiosis and reduce the risk of infections4-10 and potentially the need for some medications.7
- 1. Walsh C, et al. J Funct Foods. 2020;72:104074.
- 2. Azad MB, et al. J Nutr. 2018;148(11):1733-42.
- 3. EC No 258/97. EFSA J. 2015;13(7):4183.
- 4. Berger B, et al. mBio. 2020;11(2):e03196-19.
- 5. Puccio G, et al. J Pediatr Gastroenterol Nutr. 2017;64(4):624-31.
- 6. Roman Riechmann E, et al. Nutr Hosp. 2020;37(4):698-706.
- 7. Vandenplas Y, et al. Nutrients. 2022, 14, 530
- 8. Nowak-Wegrzyn A, et al. Nutrients. 2019;11:1447.
- 9. Boulangé CL, et al. Int. J. Mol. Sci. 2023, 24, 11422.
- 10. Gold MS, et al. Nutrients. 2022, 14, 2297
No, HMO are specific to human breastmilk (hence the name, human milk oligosaccharides). Milk from domesticated farm animals (e.g., cows) also contain “milk oligosaccharides” (MO). Human milk contains 100 to 1000 times more MO than that found in milk from domesticated farm animals, including bovine milk oligosaccharides in cow’s milk.1,2
- 1. Walsh C, et al. J Funct Foods. 2020;72:104074.
- 2. Donovan SM, & Comstock SS. Ann Nutr Metab. 2016;69(Suppl 2):42-51.
- 3. Oliveira D, et al. Int J Dairy Technol. 2015;68(3), 305-21.
Differences in lipid composition between these formulas relate to the target population of the formulas. For example, gastrointestinal impairment, which can:
• occur in combination with CMPA symptoms: Alfaré® HMO is recommended
or
• occur in those with severe CMPA symptoms: Alfamino® HMO is recommended (or Alfamino® Junior HMO when the child is >1 year)
Alfaré® HMO, Alfamino® HMO and Alfamino® Junior HMO contain medium-chain triglycerides (MCT). MCT are easily absorbed, which is useful for infants with an immature gut or intestinal failure.1,2 Althéra® HMO does not contain MCT and is indicated, as first line management, for infants with CMPA.
In addition, only Alfamino® HMO and Alfamino® Junior HMO contain structured lipids, mimicking the composition and structure of palmitic acid in breastmilk, to facilitate fat and calcium absorption.2,3
Regardless of their lipid composition, the four formulas in the range, Althéra® HMO, Alfaré® HMO and Alfamino® HMO and Alfamino® Junior HMO are suitable for the management of CMPA.
They all contain the essential fatty acids linoleic acid (LA) and α-linolenic acid (ALA), as well as the long-chain polyunsaturated fatty acids (LCPUFA), arachidonic acid (ARA) and docosahexaenoic acid (DHA), which are important for brain and immune development.2
- 1. Bach AC, & Babayan VK. Am J Clin Nutr. 1982;36(5):950-62.
- 2. Delplanque B, et al. J Pediatr Gastroenterol Nutr. 2015;61(1):8-17.
- 3. Mazzocchi A, et al. Nutrients. 2018;10(5):567.
HMO are not sensitive to heat. They also resist cold and are not affected by pasteurization and freeze-drying.1
- 1. Vandenplas Y, et al. Nutrients. 2018;10(9):1161.
No, Althéra® HMO does not contain palm oil.
2’-FL and LNnT in Nestlé infant formulas are structurally identical to those found in breastmilk.1
For decades, Nestlé has studied HMO and production has recently become technically feasible. Our range of specialty formulas, Althéra® HMO, Alfaré® HMO, Alfamino® HMO and Alfamino® Junior HMO are the first to be supplemented with both 2’-FL and LNnT.
- 1. EC No 258/97. EFSA J 2015;13(7):4183.





